Dr. Venumadhav, Dr. C. Jayakumar
Department of Pediatrics, Amrita Institute of Medical Sciences
Introduction
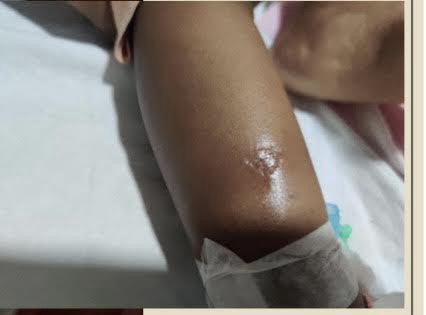
A 15-month-old female Maldivian child presented with recurrent episodes of short febrile illnesses, skin and soft tissue infections, and respiratory tract infections since early infancy. She had previously been incidentally diagnosed with neutropenia and was on treatment for the boils on her upper limbs
From 3 to 10 months of age, she experienced recurrent boils on her face, upper and lower limbs, associated with fever that requires antibiotic treatment. She was born late preterm, appropriate for gestational age (AGA), and is the third child of non-consanguineous parents.
Her neonatal course was stormy, with complications including Meconium Aspiration Syndrome and sepsis, necessitating a 10-day NICU stay.
Developmentally, she is normal and has been immunized according to the Maldivian schedule upto 6 months of age.
There is no history of chronic diarrhea, otitis media, oral ulcers, joint pains, recent weight loss, or contact with tuberculosis
EXAMINATION AND INVESTIGATIONS:
The child was afebrile with stable vitals. There was mild pallor but no icterus, cyanosis, clubbing, lymphadenopathy, or generalized edema. There were no facial dysmorphisms, skin lesions, or changes in hair or nails. Notably, there was no BCG scar.
Growth parameters were within normal limits.
Initial Differential Diagnosis considered were
1)Primary Immune deficiencies
Chronic granulomatous disease
Leukocyte adhesion deficiency
Hyper IgE syndrome
Congenital Neutropenia
2)T Cell Defects/ Bcell defects
Less severe variants of SCID
Common variable immunodeficiency
3) HIV
LAB
CBC TC 7560, Neutrophils: 1%, Absolute Neutrophil Count: 76, Lymphocytes: 76%, Absolute Lymphocyte Count: 5738, Platelets: 2.96 lakhs, Hemoglobin: 10.8).
Liver function tests (LFT) and renal function tests (RFT) were normal.
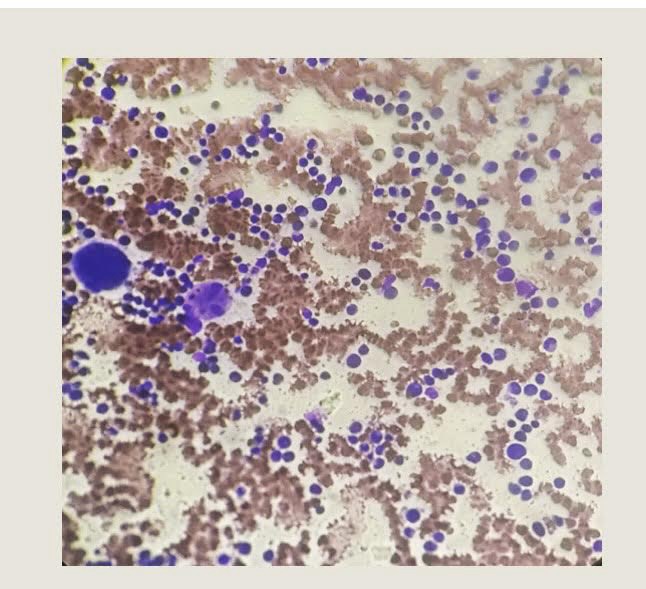
A peripheral blood smear showed normocytic normochromic anemia with neutropenia. Preop serology was negative.
Revised Differential Diagnosis Considered:
• Cyclic Neutropenia
• Kostmann Syndrome
• Schwachman-Diamond Syndrome
• Dyskeratosis Congenita
• Hermansky-Pudlak Syndrome
• Autoimmune Neutropenia of Infancy
Immunoglobulin profile was normal.
Serum folic acid and vitamin B12 levels were within normal ranges.
T/B/NK cell flow cytometry was normal.
Swab culture from the wound showed no growth.
Bone marrow aspiration revealed particulate cell marrow with trilineage maturation but reduced mature neutrophils. Bone marrow biopsy was suboptimal, showing cellular bone marrow.
Management and Outcome
After reviewing the clinical history and laboratory investigations, the primary considerations were Severe Congenital Neutropenia (SCN) and Autoimmune Neutropenia. Strict neutropenic precautions were observed, and the child was started on intravenous Clindamycin. After ruling out malignancy, she was initiated on G-CSF (Grafeel) at a dose of 5 mcg/kg/day (0.13 mL) subcutaneously, administered on alternate days after discharge until the ANC exceeded 500.
The complete blood count improved during the hospital stay, and the blisters healed. Hematopoietic stem cell transplantation was considered as a future option if the condition proved refractory to G-CSF. The child was advised to avoid all live vaccinations and to rotate injection sites.

Discussion
Severe Congenital Neutropenia (SCN), also known as Kostmann Syndrome, was first described Kostmann. It is a rare disorder with an incidence of 3-4 cases per million and no sex predilection.
Genetics and Pathogenesis: The most common cause of SCN is mutations in the genes encoding neutrophil elastase. The initial family described Kostmann had mutations in the HAX1 gene, inherited in an autosomal recessive manner. Mutations in more than 20 genes can cause SCN.
Clinical Features: Patients typically present with an absolute neutrophil count (ANC) of less than 200 and agranulocytosis. They often have oropharyngeal issues, respiratory infections, cellulitis, and skin infections, most commonly caused Staphylococci and Streptococci. These patients also have a predisposition to myelodysplastic syndrome and leukemia, predominantly acute myeloid leukemia (AML).
Treatment: G-CSF therapy (Filgrastim, Lenograstim) has dramatically improved the management of SCN, significantly reducing infection rates and enhancing the quality of life. A typical starting dose is 5 mcg/kg, which can be escalated 5 mcg/kg every 3-5 days until a response is observed.
Outcomes: A multicenter, phase 3 trial randomly assigned 123 SCN patients to either immediate treatment with Filgrastim or a 4-month observation period followed Filgrastim. Out of 108 patients, 4 were partial responders, 8 failed to respond, and the majority achieved a median ANC greater than 1500, with a 50% reduction in infections.
Challenges:
• Safety concerns with G-CSF administration, including a high frequency of osteopenia and osteoporosis.
• Patients with a suboptimal response to G-CSF face increased risks of sepsis and mortality.
Hematopoietic Stem Cell Transplantation: This potentially curative treatment should be considered for non-responders or those requiring high doses of G-CSF. Among patients treated since 2008, the overall survival rate was 80%, with chronic graft-versus-host disease occurring in 20% of cases and graft failure in 10%.
